usp class vi vs iso 10993
A further answer to a question that was partly addressed above in this thread in a manner that Im not sure was correct. In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995.

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing
Medical Silicone Rubber Molding and Silicone Rubber Mold Materials.
. Aug 05 2020 ISO 10993 and Medical Molding ISO 10993 is an international standard that divides medical devices into three categories. So does ISO 10993. 1 Acute Systemic Toxicity 2 Intracutaneous Tocixity 3 Implantation Test If one is required to adhere to ISO 10933 then the only overlap between the two test methodsregimens is the Intracutaneous Toxicity test for an ISO Class A skin contact device.
A number of our plastic materials are ISO-10993 or USP Class VI capable. Inside Rubber Magazine Profiles The Rubber Group June 28 2022. Because neither USP Class VI nor ISO 10993 are synonymous with biocompatibility testing asking for a.
Parylene has a long history of use as a protective coating for medical device biocompatibility and conforms to the USP Class VI and ISO 10993 standards. We carry a wide range of materials from the worlds top medical polymers suppliers including USP Class VI and ISO 10993 certified biocompatible resins with full FDA Master File support. While some of our rubbers can achieve this it is important to understand the customers explicit requirements.
Usp class vi versus iso 10993 search and take a look to page 8 of the ensinger_medical_brochure_for_2006. Steve Melito August 5 2020. Biocompatibility testing biocompatible materials biocompatible rubber ISO 10993 medical molder medical molding medical silicones USP Class VI.
3D printing of dental and orthopedic surgical guides. USP Class VI demands an intracutaneous irritation test. USP Class I II - Raw material supplier liability and responsibility.
Our portfolio approach offers the most expansive selection of medical resin materials in the industry balancing performance cost. Typically the terms USP Class VI or ISO 10993 materials are used. A rubber compound has set physical parameters it needs to meet.
Take an ASTM D2000 call out. A more rigorous standard for the biological evaluation of medical devices is ISO-10993. However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not.
Iso 10993 vs. This is their current stance today. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in usp class vi.
The Right Rheometer for Your Molded Rubber Parts March 30 2022. ISO 134852016 - Medical Device Quality Management Systems. The most stringent Class VI requires three types of tests.
USP Class VI vs. Other Medical Device Regulations World-Wide. That said the lack of risk assessment in USP Class VI can be a problem.
In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. In fact USP Class VI is sometimes seen as a minimum requirement for biocompatibility. The materials listed below are ideal for.
USP Class VI and ISO 10993 These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of manufacture and safety. It is transparent pin-hole free and conforms precisely to any surfaces features. My understanding is that a statement in a 510 k that a material is USP Class VI in general will not be accepted by FDA as equivalent to evidence establishing that the nominally corresponding ISO 10993.
Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. USP Class VI and ISO 10993 These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of manufacture and safety. That said the lack of risk assessment in USP Class VI can be a problem.
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a USP Class VI or even a lower USP Class certification is often sufficient. The guidance memo wasis G95-1. To begin let us address just what biocompatibility is.
Then you need to understand the differences between ISO 10993 and USP Class VI and the nature of each standard. Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by gamma irradiation and autoclave Product Validation Test Summaries available upon request Moldable bondable and formable for single-use assemblies and overmolds Temperature. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing.
USP class qualification no longer plays any role in medical device materials evaluation. While some of our rubbers can achieve this it is important to understand the customers explicit requirements. USP Class VI typically requires the following tests.
Parylenes thickness is critically controlled and extremely consistent. Class VI and ISO 10993 are recommendations for testing based on the use of the final device. Unlike other rubber standards theres no one standard that engineers use for an approval.
Biocompatibility - USP Class VI vs. Limited prolonged and permanent. Based on the time of exposure these categories are further divided into three subcategories.
Predictable Safe and Stable. Food Grade or USP Class IV Materials for Manufacturing Injectable Products. Up-to-date materials manufacturers provide both USP and ISO 10993 test data to support both pharma and device customers.
In fact usp class vi has been largely superseded since the release of iso 10993 in 1995. This post will take a deeper look at what biocompatibility is and how it is defined by the International Standards Organization. Surface implant and external communicating.
Biocompatibility Information for Materials. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts. ISO-10993 is a standard that utilizes systemic toxicity and intracutaneous reactivity testing.
How to Prevent Supply Chain Interruptions. USP class VI versus ISO 10993.

Material Selection Medical Injection Molding Xcentric Mold
Adhesives Protective Materials And Equipment For Medical Device Assembly Intertronics

Pre Colored Medical Abs Compounds For Laser Marking Plastics Technology
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
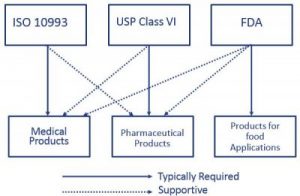
Material Selection Medical Injection Molding Xcentric Mold

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Usp Class Vi Foster Corporation

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Advantapure Apst Silicone Tubing Translucent Platinum Cured
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
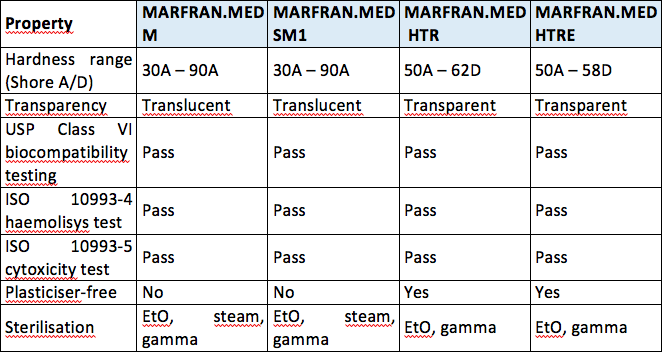
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi


